
FoundationOne Liquid CDx
See a way forward for all patients with solid tumours – using FoundationOne Liquid CDx, our FDA-approved liquid biopsy comprehensive genomic profiling service. This is a minimally-invasive option, alternative or complementary to FoundationOne®CDx at optimal times beneficial to their treatment journey.1–3
Our highly accurate and extensively validated, next-generation liquid biopsy genomic profiling service provides prognostic, diagnostic and predictive insights that inform research or treatment decisions for individual patients across all solid tumours.1,2
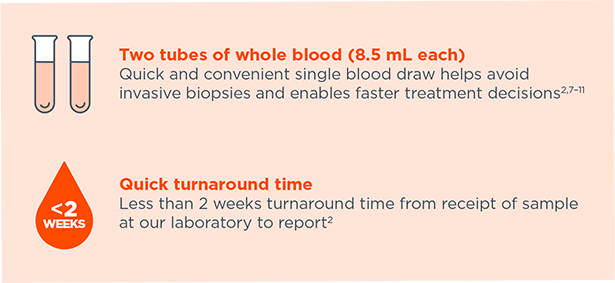
Quick and convenient single blood draw
Suitable for patients with all solid tumours
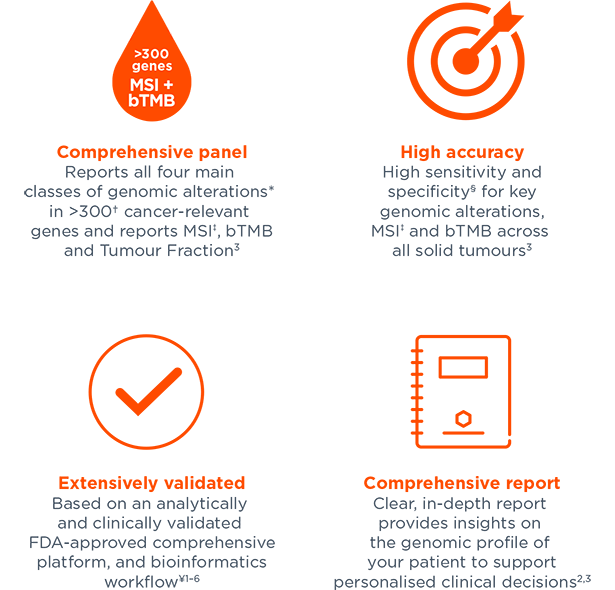
Covers guideline-recommended, and other clinically relevant genomic alterations and signatures for NSCLC, breast, ovarian and prostate cancer, among other solid tumours2,3,12–31

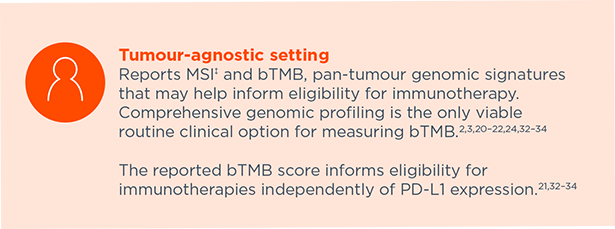
Reports MSI‡ and bTMB
Supporting personalised decision-making across the
patient journey
Part of our proven portfolio of high-quality, FDA-approved comprehensive genomic profiling services for all solid tumours, supporting current and future personalised clinical and research decisions at optimal times beneficial to your patients'
treatment journeys1−5
More information about FoundationOne Liquid CDx is coming soon. For more information about FoundationOne Liquid CDx or our portfolio of comprehensive genomic profiling services, please contact your local Roche Foundation Medicine team.
†309 genes with complete coding exonic coverage, 15 genes with select intronic or non-coding regions only.
‡FoundationOne Liquid CDx reports MSI-H status.
§75 genes are baited with enhanced sensitivity for all variant types (selected based on increased actionability with current or future targeted therapies; for more information of these 75 genes, please refer to our full gene list); other genomic regions are baited with high sensitivity.
¥Clinical validation based on evidence gathered using an earlier version of Foundation Medicine’s current liquid biopsy service, FoundationOne Liquid CDx. For concordance results between these two tests, please see our full intended use at www.foundationmedicine.com/F1LCDx.
Abbreviations:
bTMB, blood Tumour Mutational Burden; CDx, companion diagnostic; cfDNA, circulating cell-free DNA; ctDNA, circulating tumour DNA; FDA, U.S. Food and Drug Administration; MSI, Microsatellite Instability; NSCLC, non-small cell lung cancer.
- FoundationOne Liquid CDx FDA Approval, 2020. Available at: https://www.foundationmedicine.com/press-releases/445c1f9e-6cbb-488b-84ad-5f133612b721 (Accessed August 2020).
- Data on file: FoundationOne Liquid CDx Technical Specifications, 2020. Available at: http://www.eifu.online/FMI/190070862 (Accessed August 2020).
- Data on file: Clinical and analytical validation data file for FoundationOne Liquid CDx.
- FoundationOne CDx FDA Approval, 2017. Available at: https://www.fda.gov/medical-devices/recently-approved-devices/foundationone-cdx-p170019 (Accessed August 2020).
- FoundationOne CDx Technical Information, 2018. Available at: www.rochefoundationmedicine.com/f1cdxtech (Accessed August 2020).
- Gadgeel SM et al. Ann Oncol 2019; 30: S5; v851–v934; presented at ESMO 2019.
- Lebel S et al. J Psychosom Res 2003; 55: 437–443.
- Hayes et al. J Health Psychol 2017; 22: 561–571.
- Francis G, Stein. Int J Mol Sci 2015; 16: 14122–14142.
- Siravegna G et al. Ann Oncol 2019; 30: 1580–1590.
- Data on file: FoundationOne Liquid CDx Specimen Instructions.
- Planchard D et al. Ann Oncol 2019; 30: 863−870.
- NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. Version 6.2020, June 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed August 2020).
- Cardoso F et al. Ann Oncol 2019; 30: 1194−1220.
- NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 5.2020, July 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed August 2020).
- NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer. Version 2. 2020, May 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (Accessed August 2020).
- Parker C et al. Ann Oncol 2020; 31: 1119–1134.
- NCCN Clinical Practice Guidelines in Oncology. Ovarian Cancer. Version 1.2020, March 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (Accessed August 2020).
- Colombo N et al. Ann Oncol 2019; 30: 672−705.
- Khagi Y et al. Clin Cancer Res 2017; 23: 5729–5736.
- Gandara DR et al. Nat Med 2018; 24: 1441–1448.
- Abida W et al. JAMA Oncol 2019; 5: 471–478.
- Mateo J et al. Eur Urol 2017; 71: 417–425.
- Socinski M. Ann Oncol 2019; 30: v851–v934.
- Kok M et al. ESMO Open 2019; 4(Suppl 2): e000511.
- Zhao P et al. J Hematol Oncol 2019; 12: 54.
- Pikor LA et al. Lung Cancer 2013; 82: 179–189.
- Tsao AS et al. J Thorac Oncol 2016; 11: 613–638.
- Sanchez-Vega F et al. Cell 2018; 173: 321–337.e10.
- Kroeger PT Jr. et al. Curr Opin Obstet Gynecol 2017; 29: 26–34.
- Amatu A et al. ESMO Open 2016; 1:e000023.
- FDA approves pembrolizumab for adults and children with TMB-H solid tumors, 2020. Available at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (Accessed August 2020).
- Marabelle A et al. Ann Oncol. 2019;30(suppl_5):v475-v532.
- Yarchoan M et al. JCI Insight 2019; 4: e126908.