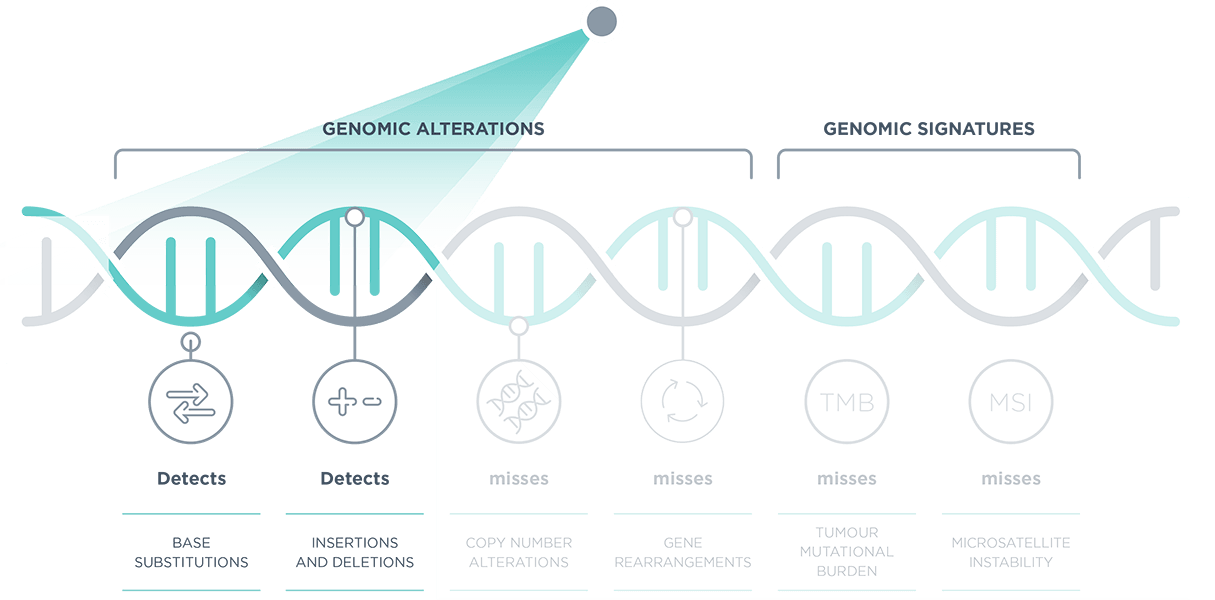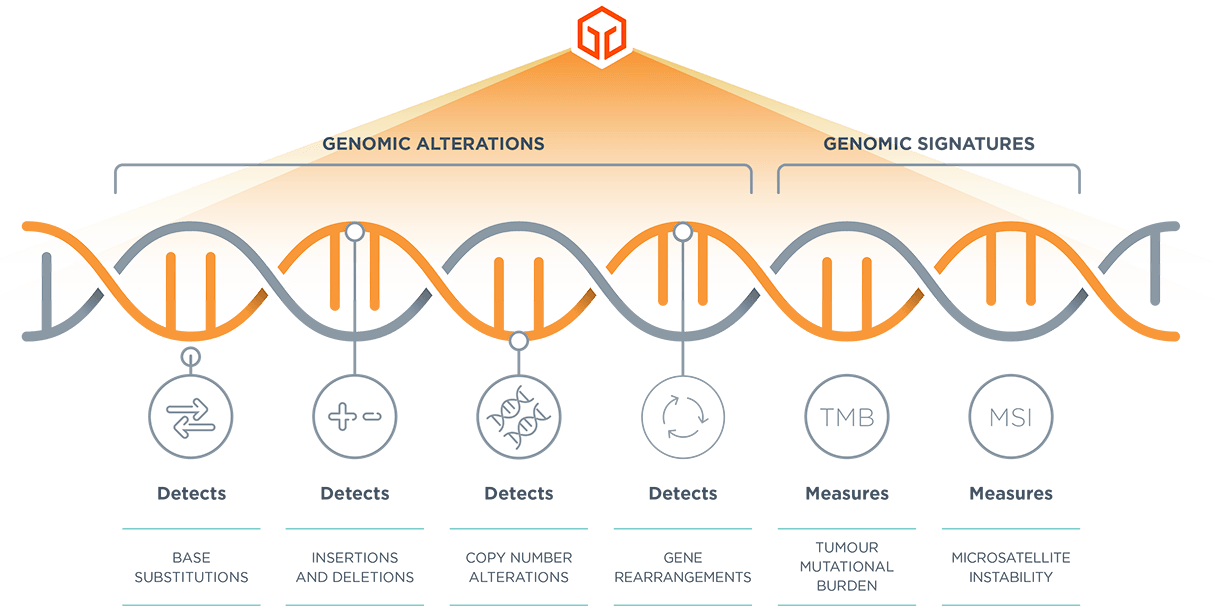
*TMB and MSI reported in FoundationOne® CDx and FoundationOne® HEME. FoundationOne® Liquid CDx reports blood TMB, MSI-high and tumour fraction.
†Therapies contained in the report may have been approved through a centralised EU procedure or a national procedure in an EU Member State.
For more information, please see the FoundationOne® CDx Technical Specifications and the FoundationOne® Liquid CDx Technical Specifications and FoundationOne® HEME Technical Specifications
CGP: comprehensive genomic profiling; EMA: European Medicines Agency; FISH: fluorescence in situ hybridisation; IHC: immunohistochemistry; MSI: microsatellite instability; NGS: next-generation sequencing; PCR: polymerase chain reaction; TMB: tumour mutational burden.
M-GB-00009116 April 2023
References
- Munoz J, et al. Am Soc Oncol Educ Book. 2013;127–134.
- Frampton GM, et al. Nat Biotechnol. 2013;31:1023–1031.
- Drilon A, et al. Clin Cancer Res. 2015;21:3631–3639.
- Rankin A, et al. Oncologist. 2016;21:1306–1314.
- Ross JS, et al. Cancer. 2016;122:2654–2662.
- Suh JH, et al. Oncologist. 2016;21:684–691.
- Hirshfield KM, et al. Oncologist. 2016;21:1315–1325.
- Schrock AB, et al. Clin Cancer Res. 2016;22:3281–3285.
- He J, et al. Blood. 2016;127:3004–3014.
- Rozenblum AB, et al. J Thorac Oncol. 2017;12:258–268.
- Woodhouse R et al. Plos One Sept 2020.
- Cheng DT, et al. J Mol Diagn. 2015;17:251–264.
- Le DT, et al. N Engl J Med. 2015;372:2509–2520.
- Rizvi NA, et al. Science. 2015;348:124–128.
- Chalmers ZR, et al. Genome Med. 2017;9:34.
- Hall MJ, et al. J Clin Oncol. 2016;30(15_suppl):1523.
- Milbury CA et al. PLoS One. 2022;17(3):e0264138.
- FoundationOne® Liquid CDx Technical Specifications.
- European Medicines Agency. Authorisation of medicines. 2018. Available at: https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines.
- 796 results, as of October 2022. Available at: https://pubmed.ncbi.nlm.nih.gov/?term=%22Foundation+Medicine%22+%5BAffiliation%5D.
- Food and Drug Administration. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Available at https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools.
- Merker JD, et al. J Clin Oncol. 2018;36:1631–1641.
- Scheerens H, et al. Clin Transl Sci. 2017;10:84–92.
- Masucci GV, et al. J Immunother Cancer. 2016;4:76.
- Milbury CA et al. PLoS One. 2022;17(3):e0264138. doi: 10.1371/journal.pone.0264138.